Pyridine Hydrochloride Pharmaceutical Intermediates Pyridinium Compounds CAS 628-13-7
Pyridine Hydrochloride Pharmaceutical Intermediates Pyridinium Compounds |
CAS No.: 628-13-7 |
Purity: >98% |
Molecular weight: 115.56 g/mol |
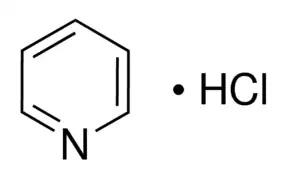
Molecular formula: C₅H₅N·HCl |
Appearance:White to white crystalline powder |
Package: 1g,10g,100g,1000g |

Pyridine Hydrochloride Pharmaceutical Intermediates Pyridinium Compounds CAS 628-13-7
Purity:98%
Package:25kg/drum
MF:C5H6ClN
Color:White to tan
Deja un mensaje
¡Te llamaremos pronto!
Querida,
Estoy interesado en Pyridine Hydrochloride Pharmaceutical Intermediates Pyridinium Compounds CAS 628-13-7, podría enviarme más detalles como tipo, tamaño, MOQ, material, etc.
¡Gracias!
Esperando su respuesta.
628-13-7 Pharmaceutical Intermediates
,98% Pharmaceutical Intermediates
,628-13-7
CAS 628-13-7 Pyridine hydrochloride Pharmaceutical intermediates Pyridinium Compounds
Description:
Pyridine hydrochloride has been widely used in the cleavage of ethers. It is shown herein that this reagent is also efficient for the synthesis of chloro compounds starting from the corresponding bromo derivatives in π-deficient series such as pyridine and quinoline. Thus, for example, 7-bromo-8-hydroxyquinoline was almost quantitatively converted into 7-chloro-8-hydroxyquinoline. The scope of the reaction has been studied.
Basic parameters:
| Synonym | PYRIDINIUM CHLORIDE |
| MW | 115.56 |
| Water solubility | 85 g/100 mL |
| Stability | Hygroscopic |
| Storage temp. | Inert atmosphere,Room Temperature |
| Melting point | 145-147 °C(lit.) |
| Boiling point | 222-224 °C(lit.) |
Chemical formula
What is Pyridine hydrochloride used for ?
This medication is used to relieve symptoms caused by irritation of the urinary tract such as pain, burning, and the feeling of needing to urinate urgently or frequently.
Is pyridine a strong base?
The availability of electron pairs represents the basicity strength. In such a case, we can say that pyridine is a strong base. The greater stability of the pyrrole is due to its ability to form a conjugated system of pi electrons.
Why is acid chloride an acid?
Acid chlorides are formed from carboxylic acids when they react with thionyl chloride. The hydroxyl group in carboxylic acid is converted to an intermediate which makes it a better leaving group, and the chloride anion functions as a nucleophile.
References:
1. Chemical book www. chemicalbook.com
2. Sciencedirect www.sciencedirect.com
3. www.vedantu.com
4. https://study.com
Recommended Products












