Lysine Cross-linked Sodium Hyaluronate Powder
Lysine Cross-linked Sodium Hyaluronate Powder |
Purity: ≥99.5%. |
Molecular weight: Typically ranges from 50,000 to 1,490,000 daltons |
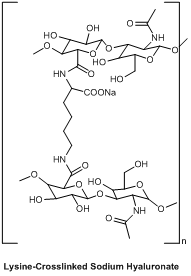
Molecular formula: C14H20NO11Na |
Appearance: white powder |
Package: 1g,10g,100g,1000g |

Lysine Cross-linked Sodium Hyaluronate Powder
Active Ingredient: Sodium Hyaluronate (cross-linked with lysine)
Description
Lysine Cross-linked Sodium Hyaluronate Powder is a sterile, biodegradable, viscoelastic hydrogel formulation. The cross-linking technology enhances the stability and longevity of hyaluronic acid (HA), providing sustained dermal volumization and tissue support. The product is reconstituted with sterile saline or lidocaine solution prior to injection.
Deja un mensaje
¡Te llamaremos pronto!
Querida,
Estoy interesado en Lysine Cross-linked Sodium Hyaluronate Powder, podría enviarme más detalles como tipo, tamaño, MOQ, material, etc.
¡Gracias!
Esperando su respuesta.
Product Name: Lysine Cross-linked Sodium Hyaluronate Powder
Active Ingredient: Sodium Hyaluronate (cross-linked with lysine)
Description
Lysine Cross-linked Sodium Hyaluronate Powder is a sterile, biodegradable, viscoelastic hydrogel formulation. The cross-linking technology enhances the stability and longevity of hyaluronic acid (HA), providing sustained dermal volumization and tissue support. The product is reconstituted with sterile saline or lidocaine solution prior to injection.
Indications
- Correction of moderate to severe facial wrinkles and folds (e.g., nasolabial folds).
- Facial contouring and volume restoration (e.g., cheeks, lips, chin).
- Dermal hydration and tissue augmentation in aesthetic medicine.
Dosage and Administration
1. Reconstitution:
- Mix the powder with the provided sterile solvent (0.9% NaCl or lidocaine HCl solution) as directed.
- Allow the gel to hydrate for 15–20 minutes.
2. Injection Technique:
- Administer intradermally or subdermally using a fine-gauge needle (27G–30G).
- Use a serial puncture or linear threading technique.
- Post-injection massage gently to ensure even distribution.
3. Dosage:
- Varies by treatment area (typically 0.1–0.4 mL per site).
- Follow clinical guidelines to avoid overcorrection.
Storage
- Store unopened powder at 2–8°C (36–46°F)**. Protect from light and moisture.
- After reconstitution, use immediately (discard unused portions).
Contraindications
- Hypersensitivity to hyaluronic acid, lysine, or lidocaine (if used).
- Active skin infection or inflammation at the injection site.
- History of severe allergic reactions (anaphylaxis).
Warnings and Precautions
- Avoid intravascular injection to prevent embolism.
- Use caution in patients with bleeding disorders or on anticoagulants.
- Delayed-onset nodules may occur; monitor patients post-treatment.
- Not recommended for use during pregnancy or lactation (limited safety data).
Adverse Reactions
Common (≥1%):
- Transient erythema, swelling, pain, or bruising at the injection site.
Rare:
- Hypersensitivity reactions (itching, rash).
- Vascular occlusion (immediate medical attention required).
Packaging
- Single-use vials containing **20 mg lysine cross-linked HA powder**.
- Packaged with sterile solvent (1.0 mL per vial).
Regulatory Status
CE Marked (for EU markets). Not FDA-approved (adjust per region).
*For medical professionals only. Use as directed. Report adverse events to local regulatory authorities.*
Recommended Products











